Abstract
Introduction: ITP is an autoimmune disease characterized by low platelet counts and variable bleeding. Ritux treatment of ITP patients (pts) receiving 375 mg/m2 once a week for 4 weeks results in 50-60% achieving complete responses (CR). However, most pts will relapse, usually around 1 year from initial treatment. In a previous study, 20 pts were retreated with a second round of 4 infusions of ritux and 16 (80%) had essentially the same response to retreatment with ritux with the second set of 4 infusions (Hasan A, AmJHem, 2009). Maintenance ritux infusions in Non-Hodgkin Lymphoma are now well-demonstrated to increase cure despite variable infusion schedules. This retrospective study explores ritux maintenance in pts with ITP or / and other autoimmune cytopenias who responded to but relapsed after a first induction with Ritux and who then received a 2nd to 4th induction with ritux.
Methods: We enrolled 17 pts with ITP, Autoimmune hemolytic anemia (AIHA), Autoimmune Neutropenia (AIN) and/or Evans syndrome, who previously responded to but then relapsed following induction with 4 ritux infusions. Ritux was administered to relapsed pts at standard dose during weeks 1, 2, 3, and 4 of induction. During the maintenance phase, single infusions of ritux were given at 1 dose of 375 mg/m2 every 4 months for a total of 2 years or until relapse or development of unacceptable adverse effects or infections or withdrawal from the study (to become pregnant); 3 pts with shorter remission times following previous ritux were treated at 3 month intervals. 5 pts received 40mg/day of dexamethasone (Dex) with the ritux maintenance infusions: 3 4-day cycles of Dex at 2 weekly intervals (Chapin, AJH, 2016) with induction and single dose dex with each maintenance infusion. The primary endpoint was the duration of response. Secondary endpoints included safety. Statistical analysis was largely descriptive. Comparisons of first and second ritux treatments (without and with maintenance) were made using the Fisher Exact test.
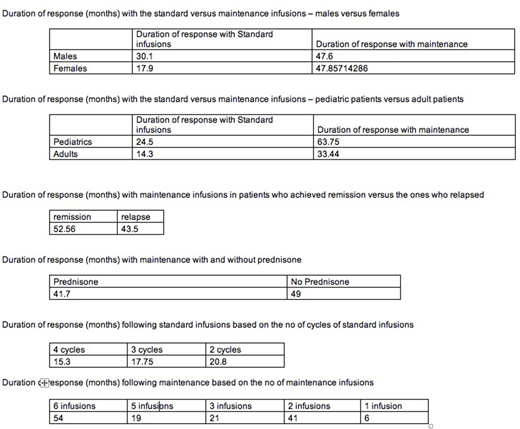
Results: Of the 17 pts who received ritux maintenance, 11 had ITP, 2 AIHA, and 4 had Evans syndrome. Three pts had AIN as part of their Evans syndrome. 7 had received more than 1 ritux induction (2-4) in the past. At initiation of maintenance, there were 10 males and 7 females and 13 adults and 4 children. Three had undergone splenectomy prior to starting ritux. The mean duration of response following the first cycle of 4 ritux standard infusions was 19 months, while the mean duration of response with maintenance schedule was 48 months. Fifteen of 17 patients achieved CR with ritux re-induction; of these 7 relapsed. 2 patients achieved PR, 1 relapsed. The mean duration of response with ritux maintenance was 36.8 months in adults and 63.8 months in children; it was 48 months each in males and females; it was 49 months in the 3 patients who had undergone splenectomy and also in those who had not undergone splenectomy. Fisher exact test did not show any statistical difference in clinical parameters including the type of autoimmune cytopenia. Pts with more previous ritux inductions did worse than those receiving their 2nd reinduction. Three patients developed hypogammaglobulinemia and needed IVIG prophylaxis against infections; one patient had bacteremia. One patient developed sepsis secondary to hypogammaglobulinemia requiring ICU admission. JC virus cultures were negative in all tested pts. No pts developed transaminitis or signs of renal failure.
Discussion: The expectation for these 17 very difficult to treat pts was that they would have all relapsed in 1 year or less following a "routine" ritux re-induction based on past data and clinical experience. The number of pts reported who achieved lasting remission following a second or third course of ritux is very small. Dex was added to reinduction in 5 of these multiply-induced pts, but curative effects occur only in female pts treated within 1 year of diagnosis, and these were pts with years of disease so this would not explain the results. Being able to achieve lasting remission over many years in half this group appears to be a significant improvement on their expected outcomes. Hypogammaglobulinemia became clinically significant in 1 pt who stopped coming for checkups; it has been reported primarily with the combination of dex-ritux but even then in only 10% of patients. A randomized controlled trial is needed to test these exciting findings.
Bussel:Prophylix: Consultancy, Research Funding; Momenta: Consultancy; Rigel: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Uptodate: Honoraria; Amgen Inc.: Consultancy, Research Funding; Protalex: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal